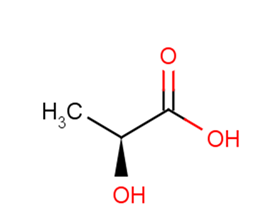
L-Lactic acid
CAS No. 79-33-4
L-Lactic acid( (S)-2-Hydroxypropanoic acid | L-(+)-Lactic acid | 2-HYDROXYPROPIONIC ACID )
Catalog No. M19665 CAS No. 79-33-4
Lactic acid is an organic acid. It is a chiral molecule consisting of two optical isomers L-lactic acid and D-lactic acid with the L-isomer being the most common in living organisms. Lactic acid plays a role in several biochemical processes and is produced in the muscles during intense activity.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 500MG | 37 | In Stock |


|
| 1G | 43 | In Stock |


|
Biological Information
-
Product NameL-Lactic acid
-
NoteResearch use only, not for human use.
-
Brief DescriptionLactic acid is an organic acid. It is a chiral molecule consisting of two optical isomers L-lactic acid and D-lactic acid with the L-isomer being the most common in living organisms. Lactic acid plays a role in several biochemical processes and is produced in the muscles during intense activity.
-
DescriptionLactic acid is an organic acid. It is a chiral molecule consisting of two optical isomers L-lactic acid and D-lactic acid with the L-isomer being the most common in living organisms. Lactic acid plays a role in several biochemical processes and is produced in the muscles during intense activity. In animals L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH) in a process of fermentation during normal metabolism and exercise. It does not increase in concentration until the rate of lactate production exceeds the rate of lactate removal. This is governed by a number of factors including monocarboxylate transporters lactate concentration the isoform of LDH and oxidative capacity of tissues. The concentration of blood lactate is usually 1-2 mmol/L at rest but can rise to over 20 mmol/L during intense exertion. There are some indications that lactate and not glucose is preferentially metabolized by neurons in the brain of several mammalian species including mice rats and humans. Glial cells using the lactate shuttle are responsible for transforming glucose into lactate and for providing lactate to the neurons. Lactate measurement in critically ill patients has been traditionally used to stratify patients with poor outcomes. However plasma lactate levels are the result of a finely tuned interplay of factors that affect the balance between its production and its clearance. When the oxygen supply does not match its consumption organisms adapt in many different ways up to the point when energy failure occurs. Lactate being part of the adaptive response may then be used to assess the severity of the supply/demand imbalance. In such a scenario the time to intervention becomes relevant: early and effective treatment may allow tissues and cells to revert to a normal state as long as the oxygen machinery (i.e. mitochondria) is intact. Conversely once the mitochondria are deranged energy failure occurs even in the presence of normoxia. The lactate increase in critically ill patients may therefore be viewed as an early marker of a potentially reversible state (PMID: 16356243 ). When present in sufficiently high levels lactic acid can act as an oncometabolite an immunosuppressant an acidogen and a metabotoxin. An oncometabolite is a compound that promotes tumor growth and survival. An immunosuppressant reduces or arrests the activity of the immune system. An acidogen is an acidic compound that induces acidosis which has multiple adverse effects on many organ systems. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of lactic acid are associated with at least a dozen inborn errors of metabolism including 2-methyl-3-hydroxybutyryl CoA dehydrogenase deficiency biotinidase deficiency fructose-16-diphosphatase deficiency glycogen storage disease type 1A (GSD1A) or Von Gierke disease glycogenosis type IB glycogenosis type IC glycogenosis type VI Hers disease lactic acidemia Leigh syndrome methylmalonate semialdehyde dehydrogenase deficiency pyruvate decarboxylase E1 component deficiency pyruvate dehydrogenase complex deficiency pyruvate dehydrogenase deficiency and short chain acyl CoA dehydrogenase deficiency (SCAD deficiency). Locally high concentrations of lactic acid or lactate are found near many tumors due to the upregulation of lactate dehydrogenase (PMID: 15279558 ). Lactic acid produced by tumors through aerobic glycolysis acts as an immunosuppressant and tumor promoter (PMID: 23729358 ). Indeed lactic acid has been found to be a key player or regulator in the development and malignant progression of a variety of cancers (PMID: 22084445 ). Lactate-induced secretion of hyaluronan by tumor-associated fibroblasts creates a milieu favourable for cell migration and metastases (PMID: 22084445 ). An acidic environment (pH 6-6.5) which is common in many tumors allows tumor cells to evade the immune response and therefore allows them to grow unchecked. Locally high concentrations of lactic acid are known to markedly impede the function of normal immune cells and will lead to a loss of T-cell function of human tumor-infiltrating lymphocytes (PMID: 22084445 ). Lactic acid is also an organic acid and acts as a general acidogen. Abnormally high levels of organic acids in the blood (organic acidemia) urine (organic aciduria) the brain and other tissues lead to general metabolic acidosis. Acidosis typically occurs when arterial pH falls below 7.35. In infants with acidosis the initial symptoms include poor feeding vomiting loss of appetite weak muscle tone (hypotonia) and lack of energy (lethargy). These can progress to heart abnormalities kidney abnormalities liver damage seizures coma and possibly death. These are also the characteristic symptoms of the untreated IEMs mentioned above. Many affected children with organic acidemias experience intellectual disability or delayed development.
-
In Vitro——
-
In Vivo——
-
Synonyms(S)-2-Hydroxypropanoic acid | L-(+)-Lactic acid | 2-HYDROXYPROPIONIC ACID
-
PathwayProteasome/Ubiquitin
-
TargetEndogenous Metabolite
-
RecptorEndogenous Metabolite
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number79-33-4
-
Formula Weight90.08
-
Molecular FormulaC3H6O3
-
Purity>98% (HPLC)
-
SolubilityDMSO:125 mg/mL (1387.66 mM)
-
SMILESC[C@H](O)C(O)=O
-
Chemical Name(S)-2-hydroxypropanoic acid
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Hoffmann G F Meieraugenstein W St?ckler S et al. Physiology and pathophysiology of organic acids in cerebrospinal fluid[J]. Journal of Inherited Metabolic Disease 1993 16(4):648-669.
molnova catalog



related products
-
2-Ketoglutaric acid
2-Ketoglutaric acid is a key molecule in the tricarboxylic acid cycleis also connected to glutamic acid and glutamine metabolisms through the transamination reactions.
-
OMDM-1
OMDM-1 is a selective and metabolically stable anandamide cellular uptake (ACU)inhibitor with a Ki of 2.4 μM.
-
H-Phe-Phe-OH
H-Phe-Phe-OH is a peptide made of two phenylalanine molecules; Phenylalanine is an essential amino acid and the precursor for the amino acid tyrosine.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


